Metrics
2024 IMPACT FACTOR
5 year Impact Factor
Eigenfactor Score
2024 CiteScore
Journal Citation Reports
(Clarivate 2025, JIF Rank)
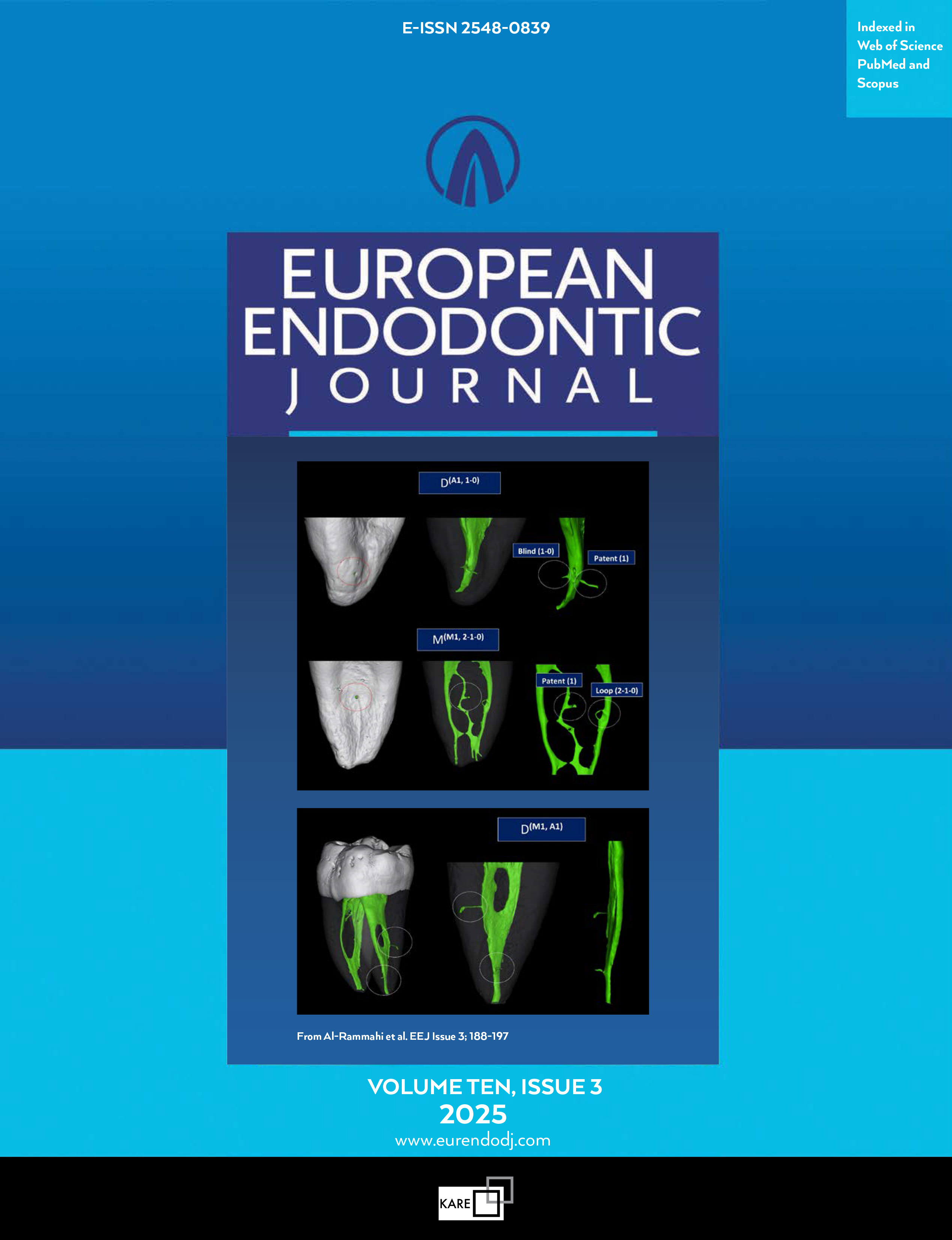
Efficiency of Immediate and Controlled Release of Aceclofenac on Post-instrumentation Pain in Root Canal Treatment A Triple Blind Randomized Controlled Trial
Jothi Latha Sundaramurthy1, Velmurugan Natanasabapathy2, Kavitha Mahendran1, Srinivasan Narasimhan3, Sandhya Raghu4, Anna Ravi Cherian2, Murugadoss Vaanjay5, Jogikalmat Krithikadatta21Department of Conservative Dentistry and Endodontics, Tamil Nadu Government Dental College and Hospital, The Tamil Nadu Dr. M.G.R. Medical University, Chennai, India2Department of Conservative Dentistry and Endodontics, Meenakshi Ammal Dental College and Hospital, Meenakshi Academy of Higher Education and Research University, Chennai, India
3Department of Endodontics, Hamad Dental Center, Hamad Medical Corporation, Doha, Qatar
4Department of Conservative Dentistry and Endodontics, Saveetha Dental College and Hospital, Chennai, India
5Department of Conservative Dentistry and Endodontics, CSI College of Dental Sciences, Madurai, India
Objective: Patients with moderate to severe preoperative pain have a high incidence of postoperative pain. The objective of this trial was to evaluate the efficiency of oral premedication with Aceclofenac (immediate release and controlled release) in the management of post-instrumentation pain in root canal treatment, in patients with moderate to severe preoperative pain.
Methods: Three-arm parallel, triple blinded randomized controlled trial was planned. Patients with moderate to severe endodontic pain, requiring primary endodontic treatment were enrolled. Aceclofenac 100mg- immediate release (Aceclofenac-IR), Aceclofenac 200mg- controlled release (Aceclofenac-CR), and Ibuprofen 400mg were compared. The tablets were given one hour before the root canal treatment.
Postoperatively, patients rated their pain at various time points. The duration of pain relief (primary outcome), the intensity of post-instrumentation pain, and the need for additional medicine were calculated. Statistical analysis was done using Kruskal-Wallis followed by Dunn post-hoc, Chi-square tests, and Binominal logistic regression.
Results: Aceclofenac-CR had a statistically significant longest duration of pain relief when compared to Ibuprofen (p=0.037) and Aceclofenac-IR (p=0.026). The intensity of post-instrumentation pain was lowest in Aceclofenac-CR, followed by Aceclofenac-IR and Ibuprofen. Additional medicine was required for only 8% of patients in Aceclofenac-CR group; whereas for 32% in each of Aceclofenac-IR and Ibuprofen groups. The odds of taking additional medicine were reduced to 0.16 in Aceclofenac-CR; increased to 1.05 with age.
Conclusion: Aceclofenac-CR had the longest duration of pain relief compared to Aceclofenac-IR and Ibuprofen. (EEJ-2022-03-037)
Manuscript Language: English
(796 downloaded)